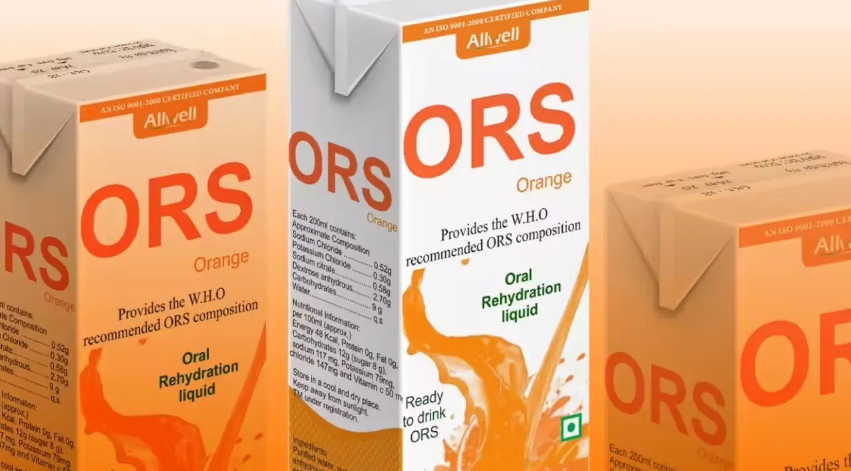
The Food Safety and Standards Authority of India (FSSAI) has announced a major directive prohibiting the use of the term “ORS” (Oral Rehydration Solution) on food product labels and advertisements across the country. The authority has instructed all business entities to comply with the order immediately.
FSSAI stated that the use of “ORS” in product names, trademarks, prefixes, or suffixes violates provisions of the Food Safety and Standards Act, 2006. Although limited use of the term was earlier allowed under notifications issued in July 2022 and February 2024, the authority has now withdrawn that permission following a recent review.
#FSSAI directs all States & UTs to ensure removal of the term “#ORS” from food products by all food business operators.
Authorities must ensure strict compliance with labelling & advertising rules under the Food Safety and Standards Act, 2006.@fssaiindia pic.twitter.com/CIVhlImTW4
— All India Radio News (@airnewsalerts) October 16, 2025
The regulator said that using the term “ORS” on products that do not meet World Health Organization (WHO) standards could mislead consumers, especially those seeking treatment for dehydration. It warned that strict legal action would be taken against any entity continuing to use the term.
All food companies have been directed to remove “ORS” from product labels, trademarks, and advertisements with immediate effect and ensure full compliance with FSSAI’s labeling and advertising rules.